

Calculate your potential savings with our ROI Calculator
ROI CalculatorNavigating the complexity of adverse event management is more than complying with stringent regulations; it’s about safeguarding what matters most—patients, customers, and product integrity. By adopting a next-generation Adverse Events Management System like Qualityze, organizations can efficiently capture, report, and resolve adverse events while ensuring compliance with evolving regulatory standards. Seamlessly integrating data, insights, and workflows, this approach helps you stay a step ahead in the journey from incident to improvement.
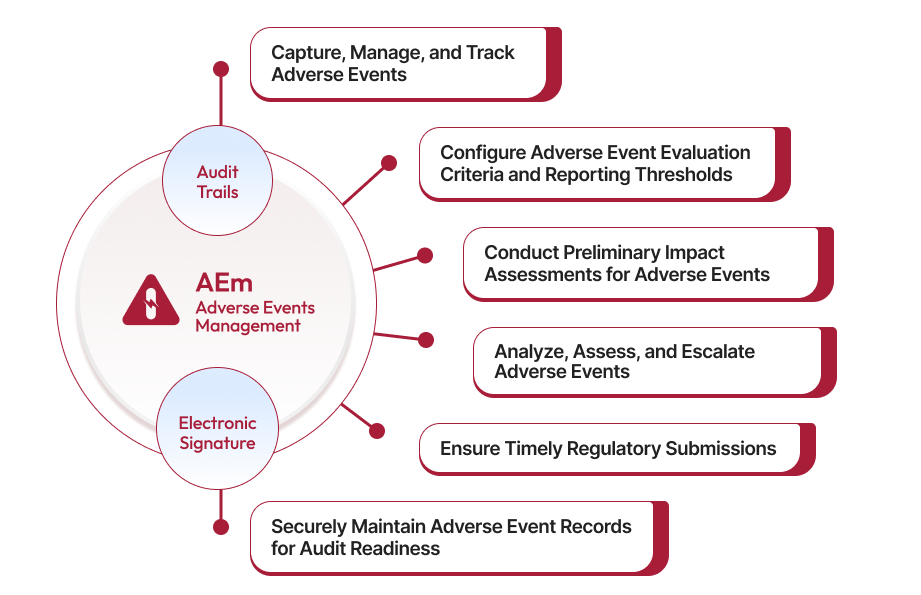
Qualityze AI Powered Adverse Event and Complaint Management Software enable organizations to systematically evaluate events based on severity and regulatory criteria for each region. With built-in guidance for every step, from evaluation to reporting, Qualityze ensures your team stays compliant, informed, and prepared, promoting a culture of transparency, accountability, and patient safety at all levels.
If you have more questions feel free to reachout to us.
Contact UsQualityze Adverse Event Management Software works by providing a cloud-based platform to capture, manage, and analyze adverse events efficiently. It streamlines workflows, helps lifesciences organizations comply with regulatory requirements, and ensures patient safety by reducing risks.
Qualityze Adverse Event Management helps organizations identify potential risks by automating data collection and analysis. It enables structured evaluation of adverse events based on severity and regional regulatory requirements, ensuring timely identification and management.
Yes, Qualityze simplifies regulatory reporting by providing clear guidance and automation for generating and submitting regulatory reports in compliance with local and international standards, such as FDA and ISO. It also makes regulatory submissions more efficient and error-free.
Qualityze Adverse Event Management aligns with global standards like FDA 21 CFR Part 820 and ISO 13485. The software automates compliance processes, ensuring organizations meet regulatory obligations consistently and effectively.
Automation helps eliminate manual data entry, reduce errors, and ensure efficient data processing. Qualityze automates adverse event data collection, risk assessment, reporting, and follow-up actions, freeing up valuable time for quality teams.
Qualityze includes features like integrated communication tools that allow team members and stakeholders to collaborate seamlessly. This helps ensure everyone is informed, leading to better decision-making and efficient handling of adverse events.
Lifesciences organizations benefit from Qualityze Adverse Event Management by gaining real-time visibility into adverse events, ensuring patient safety, maintaining regulatory compliance, reducing operational costs, and improving overall healthcare quality.
Yes, Qualityze Adverse Event Management seamlessly integrates with FDA eMDR system, automating adverse event submissions in HL7 ICSR XML format and providing real-time tracking of Ack1, Ack2, and Ack3 acknowledgments.
Qualityze provides powerful analytics tools that help identify trends and patterns in adverse event data. This supports data-driven decision-making, allowing organizations to improve their processes and prevent future adverse events.