

Calculate your potential savings with our ROI Calculator
ROI CalculatorAchieving consistent quality depends on well-calibrated equipment. Qualityze Calibration Management provides proactive calibration planning, integrated nonconformance management, and real-time insights to ensure your assets are always operating at their best, reducing equipment-related risks and enhancing overall operational efficiency.
Qualityze Calibration Management offers robust Tolerance Assessment capabilities to identify equipment that falls outside of acceptable limits. Leveraging advanced Repeatability and Reproducibility (R&R) studies, our solution helps you determine whether a Non-Conformance (NC) needs to be initiated. The R&R Study ensures minimal variations in device measurements, maintaining adherence to standard values.
If you have more questions feel free to reachout to us.
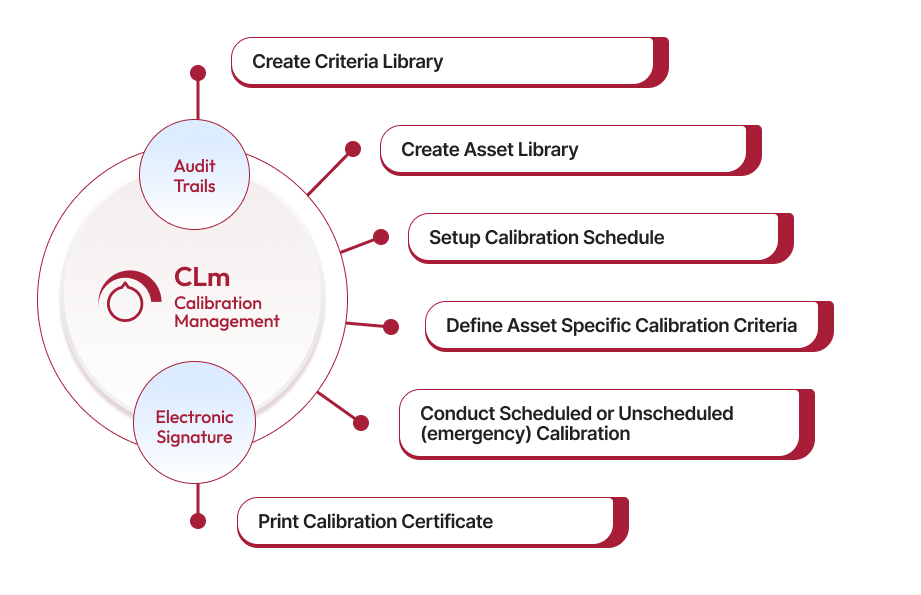
Contact UsScheduled calibration involves planned calibration activities that align with a pre-set schedule to ensure equipment accuracy and prevent unexpected issues. Unscheduled (emergency) calibration occurs when there is an urgent need to calibrate equipment, typically due to unexpected deviations or failures. Qualityze's system supports both types of calibration with alerts and predefined criteria for effective management.
When equipment is found to be 'Out of Tolerance' after calibration, Qualityze allows actions such as creating a Nonconformance (NC) report, removing the equipment from use, recalibrating, repairing, or marking it as unrepairable. The system captures details like time taken, cost incurred, asset status, and more, ensuring a comprehensive audit trail.
A calibration certificate includes the calibration status, date of calibration performed, and the next due date for calibration. Qualityze ensures these certificates are easily accessible to maintain compliance and verify that only calibrated instruments are used in production.
Yes, Qualityze Calibration Management integrates seamlessly with other quality systems, such as Nonconformance Management. This integration helps streamline calibration management, preventing product recalls and material wastage by ensuring equipment is accurately calibrated before use.
Qualityze helps minimize production downtime by enabling advanced scheduling of calibration activities, ensuring equipment is calibrated on time to prevent unexpected failures. Its integration capabilities further streamline nonconformance management, reducing potential delays.
The criteria library allows users to define calibration criteria for different equipment attributes and variables. It includes details such as criteria name, measurement values, and tolerance levels, standardizing calibration processes and ensuring equipment meets accuracy requirements.
Qualityze enables users to define asset-specific calibration criteria, including standard measurements and tolerance levels. Users can also document Repeatability & Reproducibility (R&R) studies for each asset, minimizing measurement variations and ensuring consistent quality.
Qualityze provides an asset library for capturing details like Asset ID, type, status, location, and last calibration date. Records are maintained on a centralized platform, simplifying tracking and regulatory audits.
Qualityze Calibration Management is built on a compliance-ready cloud platform, offering features like electronic signatures, audit trails, and validation packs. These tools help organizations meet ISO, FDA, and other regulatory standards by ensuring all calibration activities are well-documented and compliant.