

Regulated industries requires a consistent CAPA (Corrective and Preventive Actions) framework to eliminate systemic quality issues, from defects to nonconformances. Our AI-powered CAPA management system empowers businesses to align their workflows with proven best-practices and regulatory standards to minimize CAPAs and related risks.
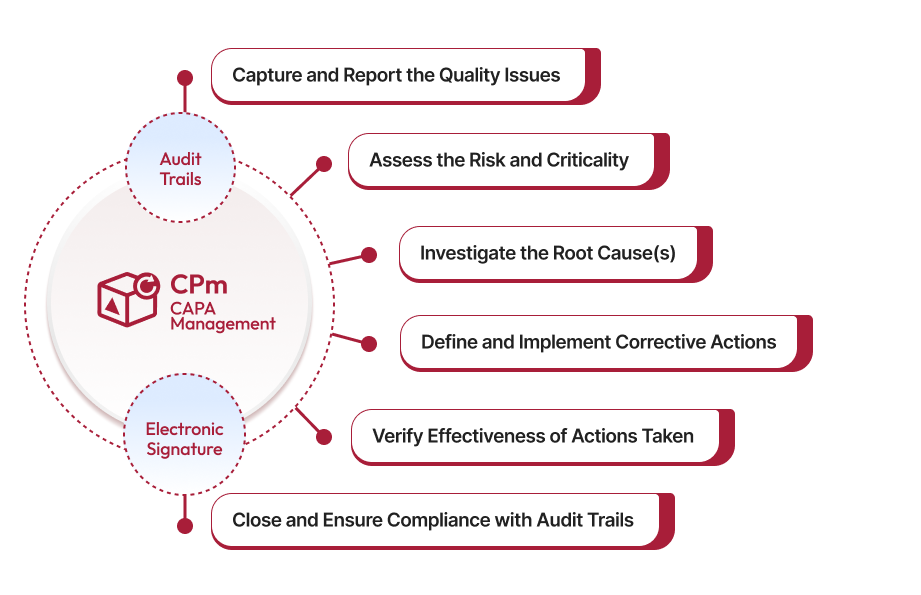
Accelerate CAPA investigations, automate workflows, and get recommendations for corrective actions with our QAI assistant. Our solution provides real-time insights into potential risks, ensuring faster resolutions, while helping you maintain regulatory compliance, and drive continuous improvement.
If you have more questions feel free to reachout to us.
Contact UsCAPA, or Corrective and Preventive Action, is a crucial process for identifying and resolving problems in quality processes, products, or workflows. It helps organizations make informed decisions about action plans. CAPA encourages:
Qualityze CAPA Management System helps you identify and eliminate root causes in your quality management system. It offers a user-friendly interface for efficient handling of CAPA processes. With this feature-packed solution, you can document quality issues, assess risks, and develop effective action plans to prevent future occurrences. However, it's important to ensure that the identified issue is a valid nonconformance for risk assessment and subsequent action implementation. Streamline your nonconformance treatment and prevent recurrence by implementing Qualityze CAPA Management Software. Request a free demo or contact our technical support for more information.
Qualityze EQMS ensures compliance by providing features that comply with industry-specific standards, such as ISO 9001, ISO 13485, 21 CFR Part 11, 21 CFR Part 820, AS9100, and IATF 16949. The solution is built on the Salesforce platform, which provides a secure and compliant cloud infrastructure. Qualityze EQMS allows businesses to maintain a complete audit trail of all quality data, ensuring regulatory compliance and reducing the risk of non-compliance.The software also provides configurable workflows that allow businesses to enforce compliance with their own internal policies and procedures. The workflows can be customized to fit specific requirements and ensure that all processes are standardized and consistent across the organization. The solution provides real-time visibility and reporting, allowing businesses to quickly identify areas of non-compliance and take corrective action. Qualityze EQMS also provides automatic version control and document management features, ensuring that all documents are up-to-date and comply with relevant regulations.
As a cloud-based solution, Qualityze EQMS is continually updated with the latest features and functionalities to ensure compliance with changing regulations and industry standards. Qualityze provides free lifetime updates to its customers to keep them up-to-date with the latest technology advancements and features. These updates are automatically applied to the system without any additional cost or disruption to the user. Qualityze’s software as a service (SaaS) model allows for seamless integration of new features and bug fixes, which means that customers always have access to the latest version of the software. Qualityze’s dedicated team of developers and quality experts continuously work on enhancing the product to ensure that it meets the evolving needs of the customers and helps them stay ahead of the competition.
Qualityze's free lifetime updates include bug fixes, security updates, feature enhancements, and new functionalities. These updates ensure that the Qualityze EQMS solution stays up-to-date and continues to meet the evolving needs of the industry and regulatory standards. The updates are provided without any additional cost to the customers and are automatically deployed to their systems. The Qualityze team ensures that the updates are thoroughly tested before being released to the customers to ensure their smooth integration and minimal disruption to their business operations. The free lifetime updates help customers to leverage the latest technological advancements and ensure that their quality management processes remain compliant and effective.
Qualityze EQMS provides a highly secure environment for managing quality data. It is built on the world’s leading cloud-based platform, Salesforce.com, which has a robust security framework with multiple layers of security features, including:
Overall, Qualityze EQMS provides a secure environment for managing quality data, giving organizations peace of mind that their sensitive data is protected.
Calculate your potential savings with our ROI Calculator
ROI Calculator